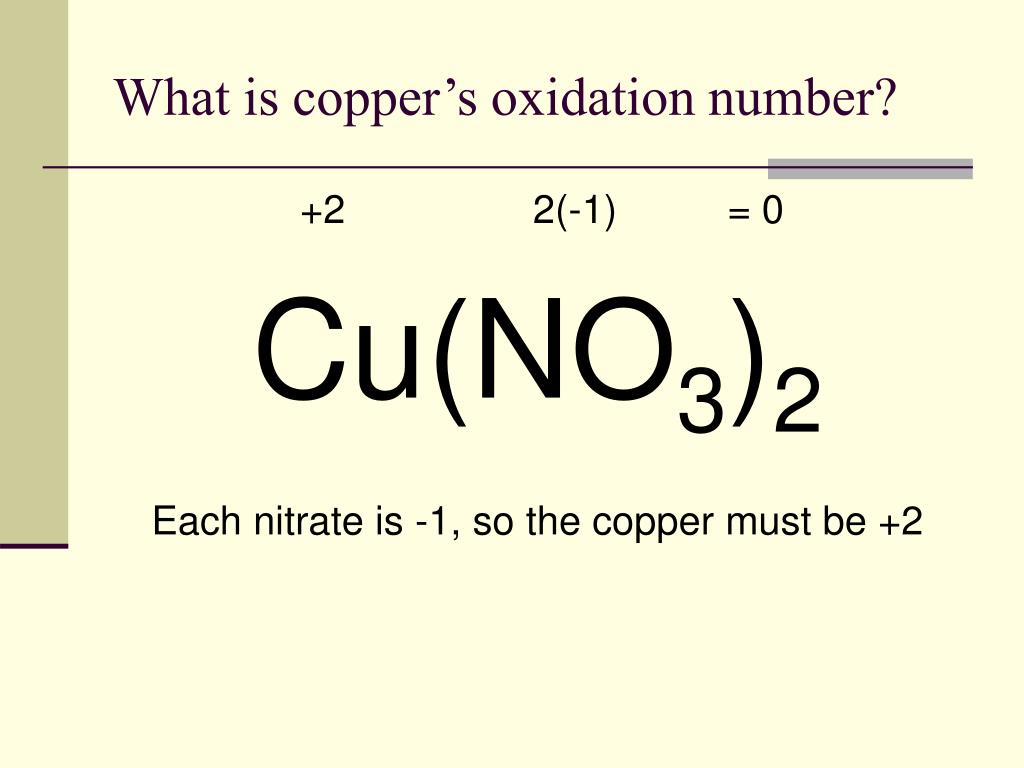Copper Chloride Oxidation . How to find the oxidation number for cu in cucl2 | copper (ii) chloride. For example, it’s often used in oxidation. Copper(ii) ions oxidize iodide ions to molecular iodine, and in the process are themselves reduced to copper(i) iodide. Copper chloride’s oxidation potential makes it a key player in many chemical reactions. Copper(ii) chloride is an inorganic chloride of copper in which the metal is in the +2 oxidation state. Copper (ii) chloride is used as a catalyst for organic and inorganic reactions, textile dyeing and printing mordant, glass and ceramic pigment,. The redox potentials $(e_{\ce{o2}} < e_{\ce{cl2}})$ suggest that oxidation of $\ce{h2o}$ to $\ce{o2}$ at the anode should.
from www.slideserve.com
Copper(ii) ions oxidize iodide ions to molecular iodine, and in the process are themselves reduced to copper(i) iodide. The redox potentials $(e_{\ce{o2}} < e_{\ce{cl2}})$ suggest that oxidation of $\ce{h2o}$ to $\ce{o2}$ at the anode should. Copper (ii) chloride is used as a catalyst for organic and inorganic reactions, textile dyeing and printing mordant, glass and ceramic pigment,. How to find the oxidation number for cu in cucl2 | copper (ii) chloride. For example, it’s often used in oxidation. Copper chloride’s oxidation potential makes it a key player in many chemical reactions. Copper(ii) chloride is an inorganic chloride of copper in which the metal is in the +2 oxidation state.
PPT Reduction Oxidation PowerPoint Presentation, free download ID
Copper Chloride Oxidation Copper(ii) chloride is an inorganic chloride of copper in which the metal is in the +2 oxidation state. How to find the oxidation number for cu in cucl2 | copper (ii) chloride. Copper(ii) chloride is an inorganic chloride of copper in which the metal is in the +2 oxidation state. Copper (ii) chloride is used as a catalyst for organic and inorganic reactions, textile dyeing and printing mordant, glass and ceramic pigment,. The redox potentials $(e_{\ce{o2}} < e_{\ce{cl2}})$ suggest that oxidation of $\ce{h2o}$ to $\ce{o2}$ at the anode should. Copper(ii) ions oxidize iodide ions to molecular iodine, and in the process are themselves reduced to copper(i) iodide. For example, it’s often used in oxidation. Copper chloride’s oxidation potential makes it a key player in many chemical reactions.
From chem.libretexts.org
3.3 Cell Potential Under Standard Conditions Chemistry LibreTexts Copper Chloride Oxidation How to find the oxidation number for cu in cucl2 | copper (ii) chloride. For example, it’s often used in oxidation. Copper(ii) chloride is an inorganic chloride of copper in which the metal is in the +2 oxidation state. Copper (ii) chloride is used as a catalyst for organic and inorganic reactions, textile dyeing and printing mordant, glass and ceramic. Copper Chloride Oxidation.
From www.pinterest.com
Electrolysing copper chloride solution to show its made of copper and Copper Chloride Oxidation The redox potentials $(e_{\ce{o2}} < e_{\ce{cl2}})$ suggest that oxidation of $\ce{h2o}$ to $\ce{o2}$ at the anode should. Copper(ii) chloride is an inorganic chloride of copper in which the metal is in the +2 oxidation state. How to find the oxidation number for cu in cucl2 | copper (ii) chloride. For example, it’s often used in oxidation. Copper (ii) chloride is. Copper Chloride Oxidation.
From www.youtube.com
Type of Reaction for Cu + HCl (Copper + Hydrochloric acid) YouTube Copper Chloride Oxidation The redox potentials $(e_{\ce{o2}} < e_{\ce{cl2}})$ suggest that oxidation of $\ce{h2o}$ to $\ce{o2}$ at the anode should. Copper(ii) chloride is an inorganic chloride of copper in which the metal is in the +2 oxidation state. For example, it’s often used in oxidation. Copper chloride’s oxidation potential makes it a key player in many chemical reactions. Copper(ii) ions oxidize iodide ions. Copper Chloride Oxidation.
From issuu.com
Reduction and oxidation copper chloride and aluminum by Fourier Copper Chloride Oxidation Copper (ii) chloride is used as a catalyst for organic and inorganic reactions, textile dyeing and printing mordant, glass and ceramic pigment,. Copper(ii) ions oxidize iodide ions to molecular iodine, and in the process are themselves reduced to copper(i) iodide. Copper chloride’s oxidation potential makes it a key player in many chemical reactions. For example, it’s often used in oxidation.. Copper Chloride Oxidation.
From blog.thepipingmart.com
Copper Oxidation States and Colors Copper Chloride Oxidation How to find the oxidation number for cu in cucl2 | copper (ii) chloride. Copper (ii) chloride is used as a catalyst for organic and inorganic reactions, textile dyeing and printing mordant, glass and ceramic pigment,. Copper(ii) ions oxidize iodide ions to molecular iodine, and in the process are themselves reduced to copper(i) iodide. Copper chloride’s oxidation potential makes it. Copper Chloride Oxidation.
From fphoto.photoshelter.com
science chemistry compound oxidation states Fundamental Photographs Copper Chloride Oxidation Copper(ii) ions oxidize iodide ions to molecular iodine, and in the process are themselves reduced to copper(i) iodide. The redox potentials $(e_{\ce{o2}} < e_{\ce{cl2}})$ suggest that oxidation of $\ce{h2o}$ to $\ce{o2}$ at the anode should. For example, it’s often used in oxidation. How to find the oxidation number for cu in cucl2 | copper (ii) chloride. Copper chloride’s oxidation potential. Copper Chloride Oxidation.
From 2012books.lardbucket.org
Oxidation and Reduction Copper Chloride Oxidation For example, it’s often used in oxidation. Copper(ii) chloride is an inorganic chloride of copper in which the metal is in the +2 oxidation state. Copper chloride’s oxidation potential makes it a key player in many chemical reactions. How to find the oxidation number for cu in cucl2 | copper (ii) chloride. Copper (ii) chloride is used as a catalyst. Copper Chloride Oxidation.
From saylordotorg.github.io
Describing Electrochemical Cells Copper Chloride Oxidation Copper(ii) chloride is an inorganic chloride of copper in which the metal is in the +2 oxidation state. For example, it’s often used in oxidation. Copper chloride’s oxidation potential makes it a key player in many chemical reactions. Copper(ii) ions oxidize iodide ions to molecular iodine, and in the process are themselves reduced to copper(i) iodide. The redox potentials $(e_{\ce{o2}}. Copper Chloride Oxidation.
From brainly.in
Benzyl alcohol on treatment with this copperbased catalyst gives a Copper Chloride Oxidation Copper chloride’s oxidation potential makes it a key player in many chemical reactions. Copper(ii) ions oxidize iodide ions to molecular iodine, and in the process are themselves reduced to copper(i) iodide. Copper (ii) chloride is used as a catalyst for organic and inorganic reactions, textile dyeing and printing mordant, glass and ceramic pigment,. For example, it’s often used in oxidation.. Copper Chloride Oxidation.
From melscience.com
Methods of copper oxidation MEL Chemistry Copper Chloride Oxidation Copper chloride’s oxidation potential makes it a key player in many chemical reactions. Copper(ii) chloride is an inorganic chloride of copper in which the metal is in the +2 oxidation state. For example, it’s often used in oxidation. Copper(ii) ions oxidize iodide ions to molecular iodine, and in the process are themselves reduced to copper(i) iodide. The redox potentials $(e_{\ce{o2}}. Copper Chloride Oxidation.
From www.youtube.com
How to find the Oxidation Number for Cu in CuCl2 Copper (II) chloride Copper Chloride Oxidation Copper (ii) chloride is used as a catalyst for organic and inorganic reactions, textile dyeing and printing mordant, glass and ceramic pigment,. Copper(ii) ions oxidize iodide ions to molecular iodine, and in the process are themselves reduced to copper(i) iodide. Copper(ii) chloride is an inorganic chloride of copper in which the metal is in the +2 oxidation state. Copper chloride’s. Copper Chloride Oxidation.
From chem.unc.edu
Chloride Oxidation by One or TwoPhoton Excitation of N Copper Chloride Oxidation Copper(ii) chloride is an inorganic chloride of copper in which the metal is in the +2 oxidation state. For example, it’s often used in oxidation. Copper chloride’s oxidation potential makes it a key player in many chemical reactions. Copper (ii) chloride is used as a catalyst for organic and inorganic reactions, textile dyeing and printing mordant, glass and ceramic pigment,.. Copper Chloride Oxidation.
From www.expii.com
Oxidizing and Reducing Agents — Definition & Examples Expii Copper Chloride Oxidation How to find the oxidation number for cu in cucl2 | copper (ii) chloride. For example, it’s often used in oxidation. Copper chloride’s oxidation potential makes it a key player in many chemical reactions. Copper (ii) chloride is used as a catalyst for organic and inorganic reactions, textile dyeing and printing mordant, glass and ceramic pigment,. The redox potentials $(e_{\ce{o2}}. Copper Chloride Oxidation.
From www.tes.com
Oxidation States and Complex half Equations Lesson AS Teaching Resources Copper Chloride Oxidation Copper chloride’s oxidation potential makes it a key player in many chemical reactions. The redox potentials $(e_{\ce{o2}} < e_{\ce{cl2}})$ suggest that oxidation of $\ce{h2o}$ to $\ce{o2}$ at the anode should. Copper(ii) chloride is an inorganic chloride of copper in which the metal is in the +2 oxidation state. Copper(ii) ions oxidize iodide ions to molecular iodine, and in the process. Copper Chloride Oxidation.
From www.compoundchem.com
Colours of Transition Metal Ions in Aqueous Solution Compound Interest Copper Chloride Oxidation Copper (ii) chloride is used as a catalyst for organic and inorganic reactions, textile dyeing and printing mordant, glass and ceramic pigment,. For example, it’s often used in oxidation. Copper(ii) chloride is an inorganic chloride of copper in which the metal is in the +2 oxidation state. Copper chloride’s oxidation potential makes it a key player in many chemical reactions.. Copper Chloride Oxidation.
From www.chemistrysteps.com
Alcohol Oxidation Mechanisms and Practice Problems Chemistry Steps Copper Chloride Oxidation How to find the oxidation number for cu in cucl2 | copper (ii) chloride. The redox potentials $(e_{\ce{o2}} < e_{\ce{cl2}})$ suggest that oxidation of $\ce{h2o}$ to $\ce{o2}$ at the anode should. For example, it’s often used in oxidation. Copper(ii) chloride is an inorganic chloride of copper in which the metal is in the +2 oxidation state. Copper chloride’s oxidation potential. Copper Chloride Oxidation.
From sites.google.com
Corrosion Year 11 Chemistry Copper Chloride Oxidation Copper (ii) chloride is used as a catalyst for organic and inorganic reactions, textile dyeing and printing mordant, glass and ceramic pigment,. For example, it’s often used in oxidation. Copper(ii) ions oxidize iodide ions to molecular iodine, and in the process are themselves reduced to copper(i) iodide. Copper chloride’s oxidation potential makes it a key player in many chemical reactions.. Copper Chloride Oxidation.
From derekcarrsavvy-chemist.blogspot.com
savvychemist Redox (II) Oxidation and Reduction Copper Chloride Oxidation Copper(ii) ions oxidize iodide ions to molecular iodine, and in the process are themselves reduced to copper(i) iodide. The redox potentials $(e_{\ce{o2}} < e_{\ce{cl2}})$ suggest that oxidation of $\ce{h2o}$ to $\ce{o2}$ at the anode should. How to find the oxidation number for cu in cucl2 | copper (ii) chloride. Copper(ii) chloride is an inorganic chloride of copper in which the. Copper Chloride Oxidation.